









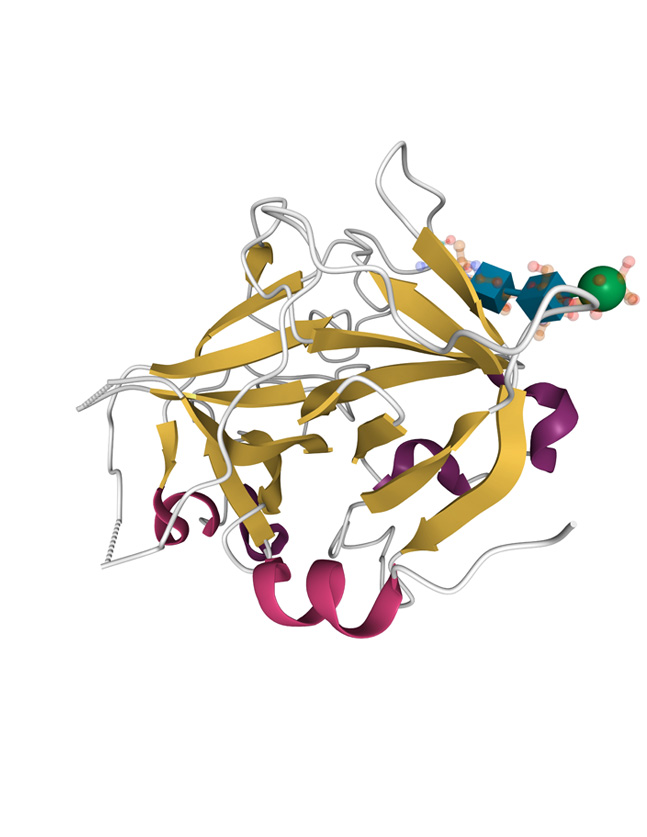
We are advancing our oral Factor XIIa inhibitor program which we believe represents a next generation therapy for the treatment of HAE and other diseases.
KalVista’s internal drug discovery and development includes an oral Factor XIIa inhibitor program which we are studying as a treatment for HAE prophylaxis. We are also exploring the potential for Factor XIIa inhibitors to address other therapeutic areas, including inflammation and thrombosis. Factor XIIa activates the kallikrein kinin system, which is an area of KalVista’s scientific leadership and oral drug discovery expertise.
There is a strong scientific rationale and positive clinical evidence for Factor XIIa inhibition in HAE prophylaxis. We believe that inhibition of Factor XIIa can be designed to block the most upstream mechanism in the contact system. Published clinical studies of Factor XIIa antibodies have demonstrated efficacy in preventing HAE attacks, and safety evaluations are ongoing for long-term inhibition of this enzyme.
See our publication with the first report of an oral, potent, and selective investigational Factor XIIa inhibitor in the peer-reviewed scientific journal, Frontiers in Pharmacology.
Learn about our science and how we target Factor XIIa to advance novel oral treatments.